VPAC1 biomarkers are expressed on cell surfaces in high densities at the onset of oncogenesis. When VPAC1 receptors are targeted, cancer can be diagnosed early, but specifically, it allows physicians to distinguish malignant from benign lesions. NuView’s new NV-VPAC1 technology works as a Positron Emission Tomography (PET) imaging agent for the in vivo diagnosis of breast and prostate cancer, by combining an NV-VPAC1 peptide with a medical imaging isotope, illuminating cancerous VPAC1 biomarkers for detection and removal. 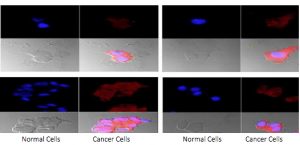
NV-VPAC1™ used in conjunction with a PET scan and fluorescent dye can detect and confirm the shed of cancer cells in voided urine, thus positioning the NV-VPAC1™ test as a non-invasive “liquid biopsy”. Unlike difficult, time-consuming, and expensive laboratory tests required to access intercellular nuclei mutations, the NV-VPAC1™ diagnostic test is uniquely positioned to confirm cancer, with its distinctive ability to visualize the over expression of the receptor on the cell membrane by binding to the VPAC receptors. If there aren’t any VPAC receptors on the cell membrane, the VPAC1 peptide is simply excreted quickly. The in vitro diagnostic test can confirm cancer cells under a pathology microscope. This could later allow for comprehensive cell confirmation of the origin of the detected cancer cells. A modified version of the test may also be used on a rapid strip test in a physician’s office to confirm the presence of cancer cells.
NuView Life Science’s new in vivo diagnostic imaging test has >95% sensitivity, which will distinguish cancerous cells non-invasively in three areas of cancer care. This will make diagnosing and treating cancer more targeted, which will help to increase positive patient outcomes and reduced biopsies, while also driving down costs incurred by patients, healthcare providers, and third-party insurance payers. Over time, NuView believes its NV- VPAC1™ test will become part of an annual screening test for millions of men and women achieving an increased potential market share by replacing outdated and less sensitive oncology tests at an attractive price to payers and improving the reliability of detecting early stage prostate cancer ultimately leads to the reduction of unnecessary surgical biopsies.
Categories: MEMBER NEWS, NuView

